Fever-range, total-body hyperthermia
- Home
- Fever-range, total-body hyperthermia
- Local Hyperthermia as a major tool in treating Cancer patients successfully in a non-toxic way
- Why are IQ scores declining over the previous 20 years?
- Expert report regarding the clinical use of CBD
- CBD effective in Healing Bone Fractures at all Ages and Prevention and Cure of Osteoporosis and Arthrosis
- Medical Cannabis Approval Sweeps Across Europe
- More people die from overdosing on pharmaceutical drugs than illegal drugs
- Flu vaccine BOMBSHELL: 630% more “aerosolized flu virus particles” emitted by people who received flu shots… flu vaccines actually SPREAD the flu
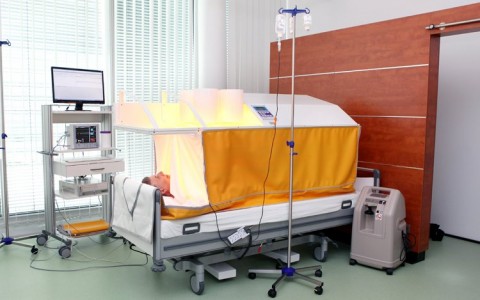
Fever-range, total-body hyperthermia has the role to restart the immune system.
Practically all cancer patients have a lower than average core temperature and are unable to develop a fever—thus they are unable to activate their immune system. To reactivate immune function, a controlled fever is induced to artificially heat the body—this process is known as therapeutic fever. Fever-range, total-body hyperthermia (or whole-body hyperthermia) is a nontoxic treatment that is often linked to improved temperature regulation in the body. The treatment is well-tolerated by practically all patients, and can be provided in combination with a range of other therapies.
How Fever Activates the Immune System
In total body hyperthermia, the patient’s core body temperature is slowly increased to about 102.2° F (39° C) and sometimes even higher to 104° F (40.0° C) if it is medical appropriate.
The immune shift is triggered by an impulse from deep within the brain stem in the hypothalamus. This area of the brain contains the command center for our most basic functions, especially those our bodies carry on without our conscious awareness such as breathing, blood pressure, and core body temperature. This command center guides the autonomic nervous system, which functions “automatically.” Fever is triggered from deep within the most ancient area of the brain, also present in all mammals, which demonstrates how fundamental fever is to our functioning.
Total-body hyperthermia treatment is used to re-launch and activate cells of the immune system. These defenses increase targeted efforts to destroy the cancer through cytotoxicity. This selective toxicity by immune cells targets malignant cells, resulting in tumor cell death (necrosis and apoptosis). Healing occurs as the cancer load is lowered through improved immune activity.
In sum, the fever primes the immune system to destroy cancerous cells and triggers increased immune responses throughout the whole body. I was diagnosed with colon cancer and that spread to my liver. Although I did well on the chemotherapy, the oncologist made it clear that treatment was palliative and not a cure. So we made an appointment for a consultation in Cologne. When we learned that Dr. Gorter’s treatment could be used together with chemotherapy, we decided to do that as well. I began the total body hyperthermia and Immune restoration therapy in Cologne.
Once you’ve received the treatment, you know what to expect. It’s not painful and I was hardly sick at all. I usually feel worse after the Immune restoration vaccination, but only for one day. Every nine weeks a scan is made and they show that there are no new tumors and the liver tumors are getting smaller. The fact that my cancer has stabilized is remarkable. (Truus Kleij-Swan, more than five year long-term survivor of intestinal cancer, metastasized to the liver, lymph nodes and peritoneum.)


 Deutsch
Deutsch Nederlands
Nederlands Turkish
Turkish Russian
Russian Italiano
Italiano Français
Français Português
Português العربية
العربية